INTRODUCTION
Multiple myeloma disproportionately impacts ethnic and racial minority groups, affecting both incidence and outcome. There is limited evidence-based data for clinical decision making for minority populations, including Black patients (Habr and Corsaro, Hematol Oncol 2022).
Elranatamab is a B-cell maturation antigen (BCMA)-CD3 bispecific antibody. In the phase 2 registrational trial MagnetisMM-3, elranatamab demonstrated clinical efficacy in BCMA-naïve and BCMA-exposed patient populations. Here, we report the efficacy and safety of elranatamab in a pooled analysis of Black or African American patients with RRMM across 3 studies in the MagnetisMM program, including MagnetisMM-1 (MM-1; NCT03269136), MagnetisMM-3 (MM-3; NCT04649359), and MagnetisMM-9 (MM-9; NCT05014412).
METHODS
Eligible patients included in this pooled analysis had previously received at least 1 proteasome inhibitor, 1 immunomodulatory drug, and 1 anti-CD38 antibody, and were either naïve or exposed to prior BCMA-directed therapy (antibody-drug conjugate [ADC] and/or CAR T-cells). All patients received subcutaneous elranatamab at the effective doses of 215-1000 µg/kg or fixed dose equivalent of 76 mg (MM-1) or 76 mg once-weekly (MM-3 and MM-9). Treatment continued until disease progression, unacceptable toxicity, or withdrawal of consent. Evaluation of overall response rate (ORR) was assessed per IMWG criteria. Adverse events (AEs) and laboratory abnormalities were graded by National Cancer Institute Common Terminology Criteria for AEs v4.03 (MM-1) or v5.0 (MM-3 and MM-9). American Society for Transplantation and Cellular Therapy criteria (Lee DW, et al. Biol Blood Marrow Transplant 2019) were used for grading cytokine release syndrome (CRS) and immune effect cell-associated neurotoxicity syndrome (ICANS).
RESULTS
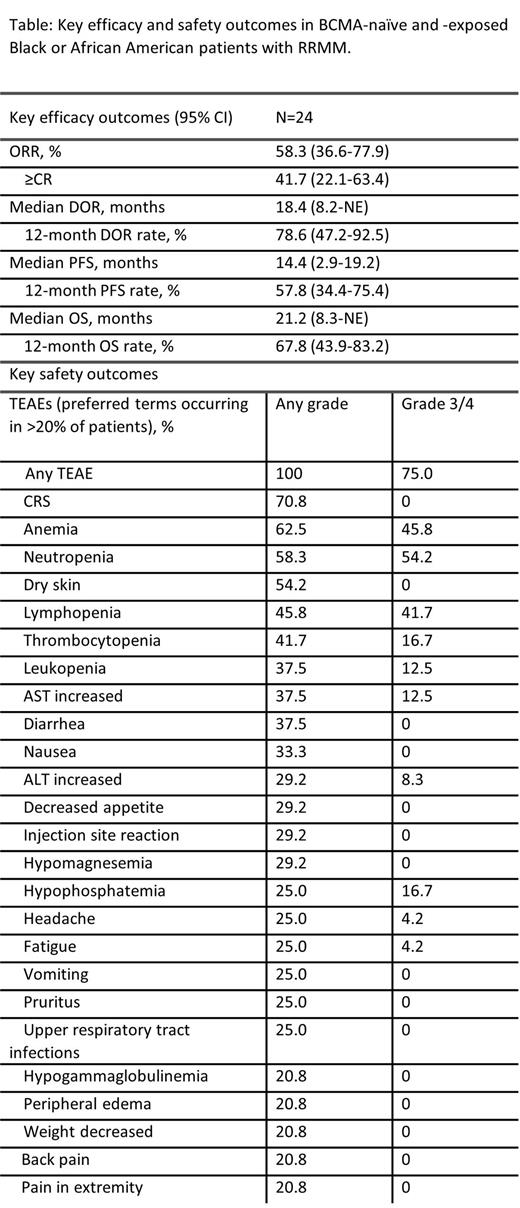
Of 24 Black or African American patients in the pooled analysis who received elranatamab, the median age was 61.5 years (36-76), 75.0% had R-ISS Stage I or II disease, 79.2% had prior stem cell transplant, and 91.7% patients had an Eastern Cooperative Oncology performance status of 0 or 1. Patients had a median of 6 prior lines of therapy (range, 2-14), with 91.7% and 45.8% having triple-class and penta-drug refractory disease, respectively. Seven patients (29.2%) received prior BCMA-directed therapy as either an antibody-drug conjugate (ADC; n=5), CAR-T (n=4), or both (n=2). After a median follow-up of 13.3 months, the median duration of treatment was 9.0 months; 6 (25%) patients were still on treatment. The most common reasons for treatment discontinuation were progressive disease (41.7%), AEs (12.5%), and death (12.5%). The ORR was 58.3%, with 41.7% of patients achieving complete responses (CR) or better (Table). The median duration of response (DOR), progression-free survival (PFS), and overall survival (OS) were 18.4, 14.4, and 21.2 months, respectively. Common treatment-emergent AEs (TEAEs) included CRS, anemia, and neutropenia (Table). Infections were reported in 15 patients (any grade, 62.5%; grade 3/4, 20.8%). Two cases of ICANS (both grade 2) were reported. One patient died due to disease progression (metastases to meninges), and four patients died due to a TEAE other than disease progression: 1 patient each due to COVID-19, septic shock, sudden death, and pulmonary embolism.
CONCLUSIONS
Elranatamab demonstrated clinical activity and a manageable safety profile in Black or African American patients with RRMM, supporting the use of elranatamab in this subgroup of patients. Efficacy and safety results are comparable to those observed for the overall population of patients treated with elranatamab in the phase 2 MagnestisMM-3 trial. No new safety signals were observed.
OffLabel Disclosure:
Varshavsky-Yanovsky:Pfizer: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees. Nooka:Aduro Biotech, Amgen, Arch Oncology, Bristol Myers Squibb, Cellectis, Genentech, GlaxoSmithKline, Janssen, Karyopharm, Kite Pharma, Merck, Pfizer, Takeda: Honoraria, Research Funding; Adaptive Biotechnologies, Amgen, BeyondSpring, Bristol Myers Squibb, Cellectar Biosciences, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, ONK therapeutics, Pfizer, Sanofi, Secura Bio, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Stiff:Takeda: Research Funding; Macrogenics: Research Funding; Incyte Corp: Research Funding; Gamida Cell: Research Funding; Eisai: Research Funding; AtaraBiotherapeutics: Research Funding; Amgen: Research Funding; CRISPR: Consultancy. Perez-Cruz:Pfizer: Current Employment. Leip:Pfizer: Current Employment. Lesokhin:Iteos: Consultancy; BMS: Research Funding; Janssen: Research Funding; Genentech: Research Funding; Serametrix (now Caprion): Patents & Royalties; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Arcellx: Membership on an entity's Board of Directors or advisory committees.
Elranatamab is an investigational BCMA-CD3 bispecific antibody for the treatment of multiple myeloma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal